Investors claim Philips’ faulty sleep apnea machines cost them €16bn
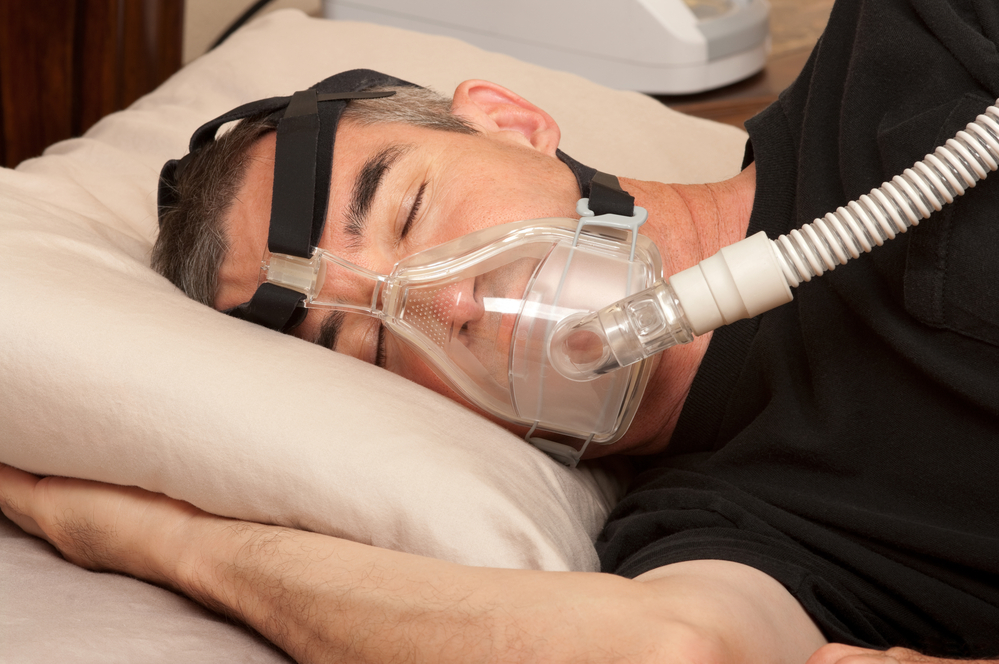
Investors in technology firm Philips have launched a claim for €16 billion for allegedly being misinformed about the extent of problems with the company’s devices to treat sleep apnea.
Philips has recalled 5.5 million machines and 17 million sleep masks worldwide after it emerged that tiny foam particles could be released during cleaning and then inhaled, while magnetic clips in the masks potentially interfere with heart pacemakers.
The replacement of the Philips Respironics ventilators has cost the company an estimated €900 million and driven down its share price by more than 60% in the last 18 months.
Investors’ association VEG said its members, as well as sleep apnea patients, had been ‘systematically misinformed’ about the problems with the breathing devices, and as a result that the company’s share price was artificially high.
The VEB claimed that research by the Food and Drug Administration, which regulates the sale and distribution of medicines in the USA, showed that the company had been aware of the defects in the foam masks since 2015.
The organisation also criticised Philips’s CEO Frans van Houten, who is standing down next month, for his claim that senior executives had only known about the problems with the machines since 2021.
‘Processes failed’
‘They could have known about it if their checking procedures were in order,’ Gerben Everts, chair of the VEB, told the Financieele Dagblad. ‘The internal processes failed. That is very serious and the management is culpable for it.’
The VEB sent a formal letter to Philips at the weekend stating it holds the company liable for the devaluation of its members’ assets and gave the company two weeks to begin talks to negotiate a compensation package. If no offer is made, the group intends to go to court.
Philips has said it does not expect to have to pay any compensation and has commissioned several reports in the last few months showing that the risk to patients is minimal because the masks only degrade when cleaned with an aggressive method that is rarely used in the EU or the US.
However, the FDA said it had received 14 reports of injuries, including heart arrhythmia, seizures and pacemaker failure, that may be linked to use of its machines. It has urged patients and caregivers to replace their machines because of a ‘serious safety concern’.
A spokesman for Philips confirmed it had received a letter from the VEB about its ‘field safety notice’ from June 2021. ‘We are convinced that Philips has acted adequately and with integrity, and we are confident about a possible discussion with the VEB regarding this field safety notice,’ he said in a written response. ‘We will first study the content of the letter in more detail, before we provide any further comments.’
Thank you for donating to DutchNews.nl.
We could not provide the Dutch News service, and keep it free of charge, without the generous support of our readers. Your donations allow us to report on issues you tell us matter, and provide you with a summary of the most important Dutch news each day.
Make a donation