Womed Launches Female Fertility Preserving Device in Europe
Womed Leaf®, the First Intrauterine Adhesion Barrier Film, to be distributed in 14 European Countries
MONTPELLIER, France–(BUSINESS WIRE)–#femtech–Womed, the uterine health company developing innovative, safe and effective treatments to free women from uterine pathologies, today announced it has signed licensing agreements with Kebomed Europe and Saesco Medical to distribute its first medical device, Womed Leaf®, in Europe.
Intrauterine Adhesions (IUA), which refer to the pathological binding of the uterine walls, are caused by scarring of the uterus after procedures such as dilation and curettage or fibroid removal, and can occur in 20% to 45% of those procedures. IUAs are a major cause of infertility, recurrent miscarriages and pain. IUA treatment is plagued with a very high recurrence rate, leaving women unsure and very anxious about their chance to conceive.
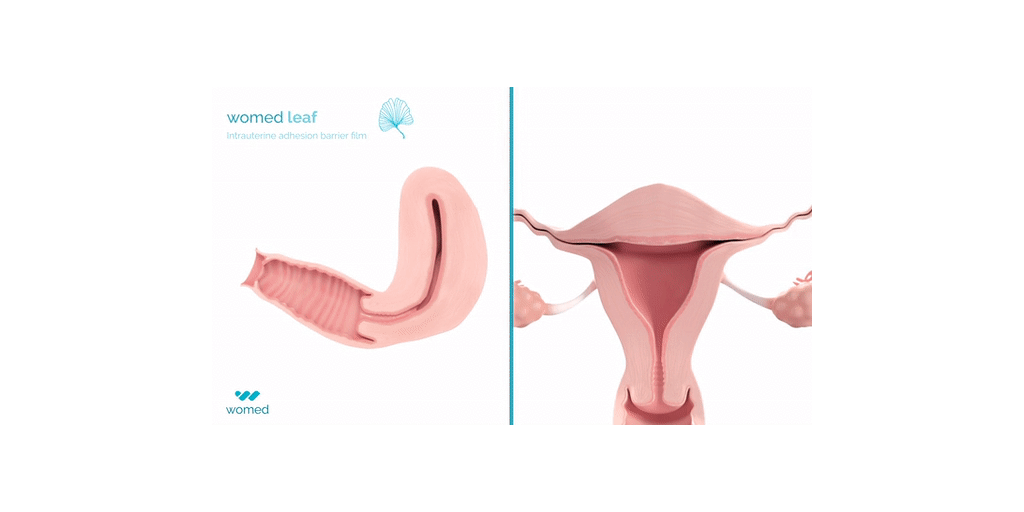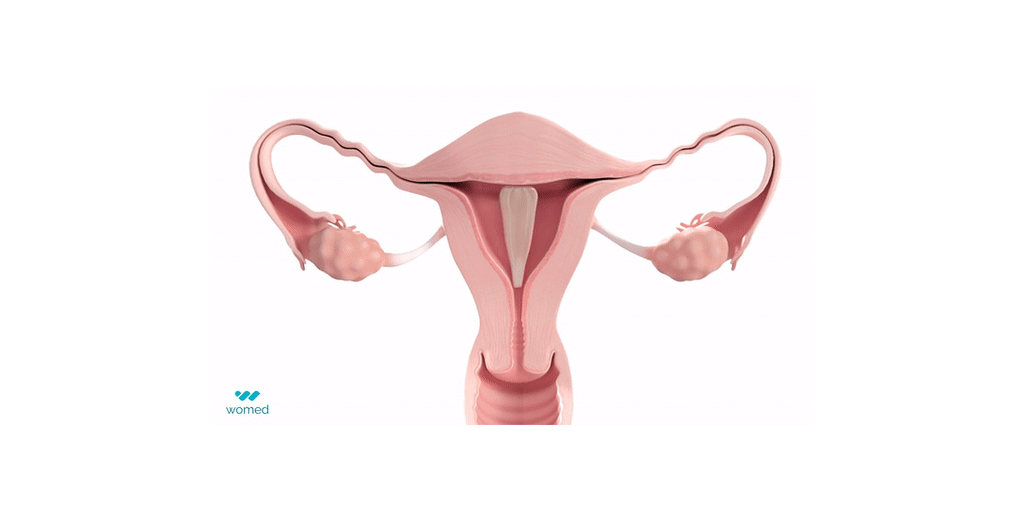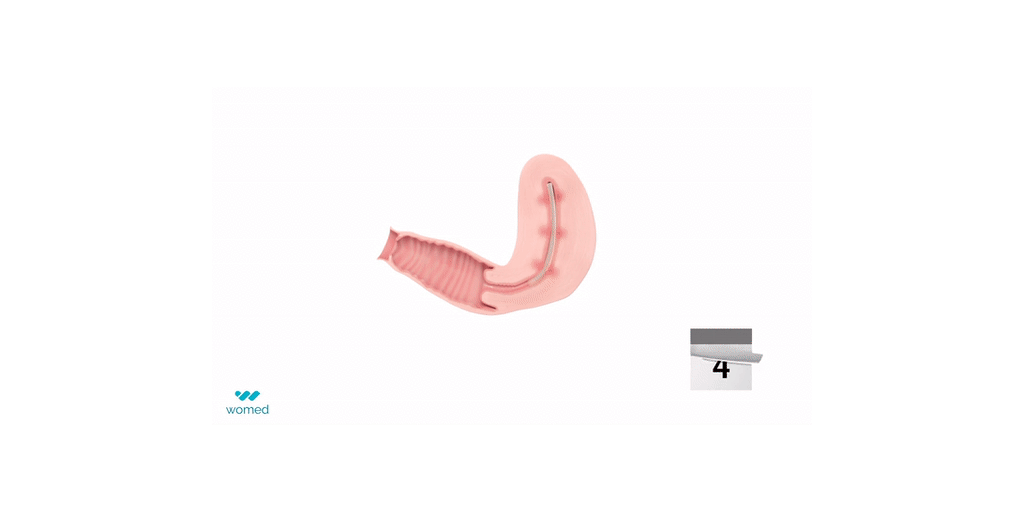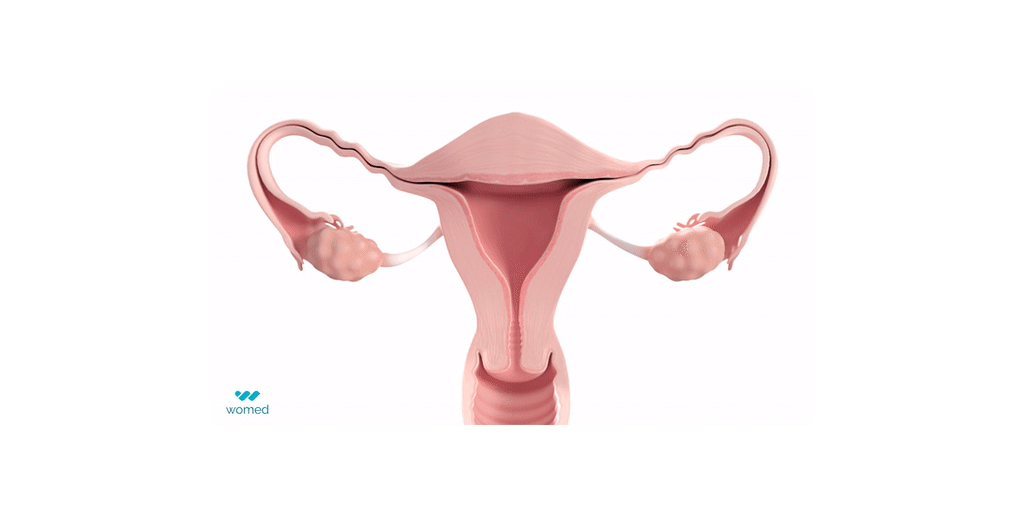
Womed Leaf® is the first and only physical barrier to protect against IUA. The device consists of a soft, flexible film made from Womed’s innovative polymer, which is inserted like an IUD at the end of a uterine procedure. It expands within the cavity, preventing contact between the uterine walls for one week, and is then naturally and painlessly discharged. Womed Leaf was proven to be highly effective in the PREG2 randomized clinical study that enrolled 160 patients with severe or moderate IUA.
“These partnerships mark the first step of a global launch that will make Womed Leaf the new gold standard in intrauterine adhesion prevention and treatment,” said Gonzague Issenmann, Co-founder and CEO of Womed. “Kebomed Europe and Saesco Medical’s established sales networks and expertise in women’s health will help European women and their doctors to preserve or recover their fertility.”
“Womed Leaf addresses a significant unmet need in women’s health and we are excited to partner with Womed to bring it to our customers,” said Lars Melbye, Managing Director of Kebomed Europe. Jordi Fluvia, CEO of Saesco Medical, added: “We are committed to offering the latest advances in medical technologies, and Womed is bringing a true innovation in an indication that has been sorely lacking in meaningful improvements for the last two decades.”
Kebomed Europe will have the responsibility of selling the device in France, Germany, Sweden, Denmark, Norway, Finland, Austria and Switzerland, while Saesco Medical will cover Spain, Italy, Portugal, Belgium, the Netherlands and Luxembourg.
Staatz Business Development & Strategy served as the business development partner to Womed.
About WOMED
Womed is the uterine health company developing products based on its disruptive, proprietary, polymer technology platform, designed for intrauterine implantation and local active ingredient administration. Its first product, Womed Leaf®, is a medical device to treat and prevent bonding of the uterine walls, which occurs in particular in one in five women treated for miscarriage. Womed’s pipeline of intrauterine drug delivery products include treatments for fibroids, endometriosis and acute uterine bleeding.
www.womedtech.com
About Kebomed Europe
Kebomed Europe is a leading independent distributor of medical devices specializing in the field of women’s health. Kebomed Europe partners with manufacturers from around the world to bring innovative medical technologies to healthcare providers and improve patient care.
www.kebomed.com
About Saesco Medical
Saesco Medical is a distributor of medical devices. The company is dedicated to providing high-quality medical equipment and supplies to healthcare providers.
www.saescomedical.com
Contacts
Press contact:
Gonzague Issenmann, CEO, Womed
news@womedtech.com
Thank you for donating to DutchNews.nl.
We could not provide the Dutch News service, and keep it free of charge, without the generous support of our readers. Your donations allow us to report on issues you tell us matter, and provide you with a summary of the most important Dutch news each day.
Make a donation